Опубликована статья: Bidusenko I.A., Schmidt E.Yu., Ushakov I.A., Vashchenko A.V., Trofimov B.A. Base-catalyzed [3+2] cycloaddition of N-benzyl ketimines to arylacetylenes followed by oxidation: a one-pot access to polyarylated 2H-pyrroles via intermediate pyrrolines // Organic Letters. – 2021. – V. 23. – Iss. 11. – P. 4121-4126. IF 6,005. Q1 (БАЦКП, РНФ 19-13-00052) 04.06.2021. DOI: 10.1021/acs.orglett.1c01009
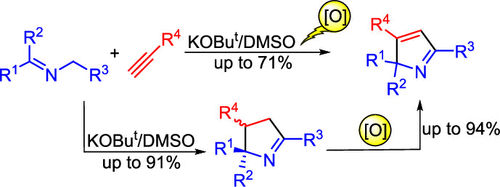
Abstract: N-Benzyl ketimines undergo [3 + 2] cycloaddition with arylacetylenes in the KOBut/DMSO solution to 2,3,5-triarylpyrrolines, which are oxidized (chloranil, DDQ) in situ to 2,3,5-triaryl-2H-pyrroles in 53–71% yields. The intermediate 1-pyrrolines can be isolated in 31–91% yields and separately oxidized to the corresponding 2H-pyrroles.
Поздравляем авторов статьи и желаем дальнейших успехов!
Пресс-центр ИрИХ СО РАН















